Abstract
Introduction
Melphalan flufenamide (melflufen), is a novel peptide-drug conjugate that targets aminopeptidases and selectively delivers alkylating agents in tumors. Melflufen was recently FDA approved for the treatment of relapsed/refractory multiple myeloma (MM) patients. Considering the challenges in treating this group of patients, and the availability of several new drugs for MM, information that can support treatment selection is urgently needed. To identify potential indicators of response and mechanism of resistance to melflufen, we applied a multiparametric drug sensitivity assay to MM patient samples ex vivo and analyzed the samples by single cell RNA sequencing (scRNAseq). Ex vivo drug testing identified MM samples that were distinctly sensitive or resistant to melflufen, while differential gene expression analysis revealed pathways associated with response.
Methods
Bone marrow (BM) aspirates from 24 MM patients were obtained after written informed consent following approved protocols in compliance with the Declaration of Helsinki. BM mononuclear cells from 12 newly diagnosed (ND) and 12 relapsed/refractory (RR) patients were used for multi-parametric flow cytometry-based drug sensitivity and resistance testing (DSRT) evaluation to melflufen and melphalan, and for scRNAseq. Based on the results from the DSRT tests and drug sensitivity scores (DSS), we divided the samples into three groups - high sensitivity (HS, DSS > 40 (melflufen) or DSS > 16 (melphalan)), intermediate sensitivity (IS, 31 ≤ DSS ≤ 40 (melflufen) or 10 ≤ DSS ≤ 16 (melphalan)), and low sensitivity (LS, DSS < 31 (melflufen) or DSS < 10 (melphalan)). To identify genes, responsible for the general sensitivity to melphalan-based drugs we conducted differential gene expression (DGE) analyses separately for melphalan and melflufen focusing on the plasma cell populations, comparing gene expression between HS and LS samples for both drugs ("HS vs. LS melphalan" and "HS vs. LS for melflufen", respectively). In addition, to explain the increased sensitivity of RR samples, we conducted the DGE analysis for ND vs. RR samples and searched for similarities between these three datasets.
Results
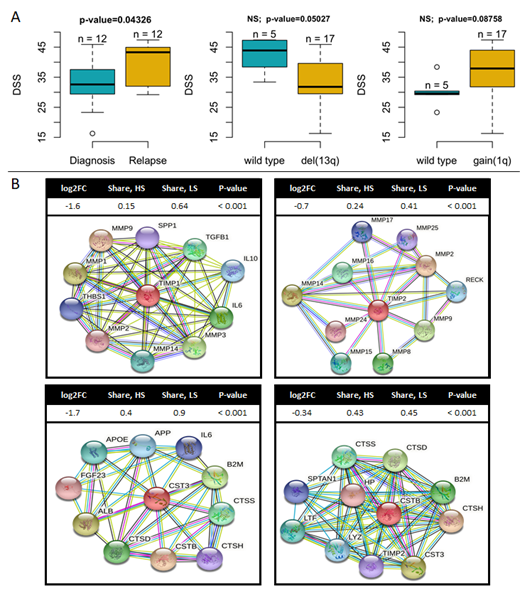
DSRT data indicated that samples from RRMM patients were significantly more sensitive to melflufen compared to samples from NDMM (Fig. 1A). In addition, we observed that samples with a gain of 1q (+1q) were more sensitive to melflufen while those with deletion of 13q (del13q) appeared to be less sensitive, although these results lacked significance (Fig. 1A). After separating the samples into different drug sensitivity groups (HS, IS, LS), DGE analysis showed significant downregulation of the drug efflux and multidrug resistance protein family member ABCB9 in the melflufen HS group opposed to the LS group (2.2-fold, p < 0.001). A similar pattern was detected for the melphalan HS vs. LS comparison suggesting that this alteration might be a common indicator of sensitivity to melphalan-based drugs. Furthermore, in the melflufen HS group we observed downregulation of the matrix metallopeptidase inhibitors TIMP1 and TIMP2 (3-fold and 1.6-fold, p < 0.001, respectively), and cathepsin inhibitors CST3 and CSTB (3.2-fold and 1.3-fold, p < 0.001, respectively) (Fig. 1B). This effect was observed in both "ND vs. RR" and "HS vs. LS for melflufen" comparisons, but not for melphalan, suggesting that these changes are associated with disease progression and specific indicators of sensitivity to melflufen. Moreover, gene set enrichment analysis (GSEA) showed activation of pathways related to protein synthesis, as well as amino acid starvation for malignant and normal cell populations in the HS group.
Conclusion
In summary, our results indicate that melflufen is more active in RRMM compared to NDMM. In addition, samples from MM patients with +1q, which is considered an indicator of high-risk disease, tended to be more sensitive to melflufen. Based on differential GSEA and pathway enrichment, several synergizing mechanisms could potentially explain the higher sensitivity to melflufen, such as decreased drug efflux and increased drug uptake. Although these results indicate potential indicators of response and mechanisms of drug efficacy, further validation of these findings is required using data from melflufen treated patients.
Slipicevic: Oncopeptides AB: Current Employment. Nupponen: Oncopeptides AB: Consultancy. Lehmann: Oncopeptides AB: Current Employment. Heckman: Orion Pharma: Research Funding; Oncopeptides: Consultancy, Research Funding; Novartis: Research Funding; Celgene/BMS: Research Funding; Kronos Bio, Inc.: Research Funding.